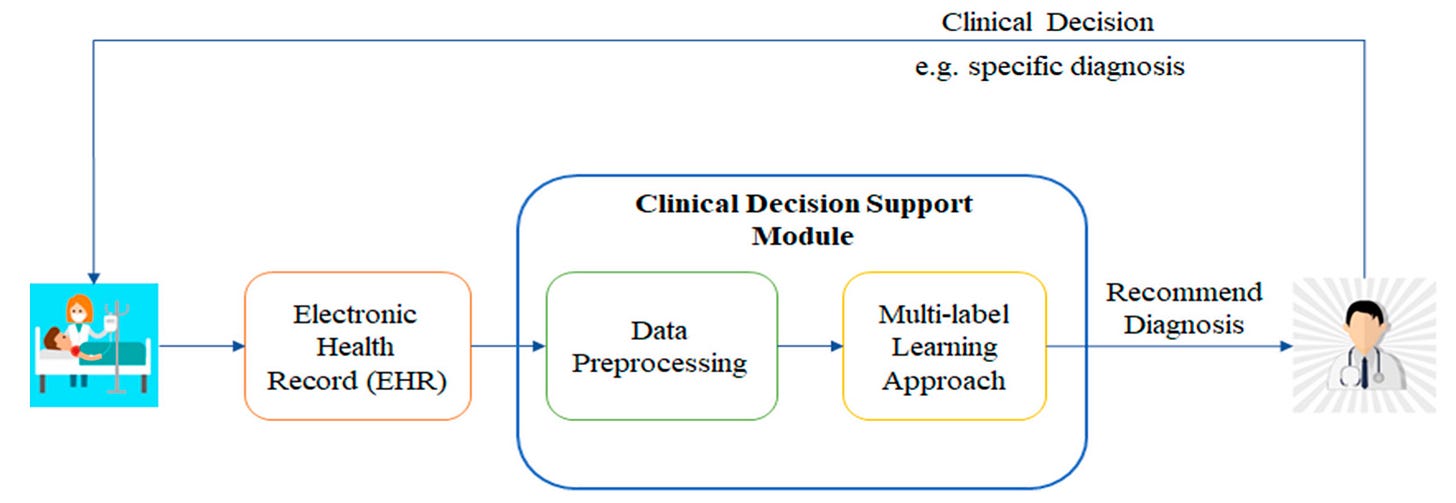
Rethinking Clinical Decision Support in the Era of Foundation Models: From Alert Fatigue to Intelligent Inference
Disclaimer: The thoughts and opinions expressed in this essay are my own and do not reflect those of my employer.
Abstract
Clinical Decision Support systems have reached an inflection point. After decades of rule-based alerts that have created more noise than signal, foundation models present an unprecedented opportunity to reimagine how we augment clinical reasoning. This essay examines the technical and practical challenges of integrating large language models and multimodal foundation models into CDS pipelines, moving beyond the tired paradigm of alert fatigue toward systems that genuinely enhance clinical workflows. We explore critical system design decisions including on-premises versus cloud inference architectures, governance frameworks for managing model drift, and integration strategies within existing EHR ecosystems. The essay addresses practical implementation challenges such as achieving sub-200ms latency for retrieval-augmented generation with local clinical guidelines, while maintaining the reliability and safety standards required in healthcare environments.
Table of Contents
1. Introduction: The Promise and Peril of Intelligent CDS
2. The Foundation Model Revolution in Healthcare
3. System Architecture: Cloud vs Edge in Clinical Environments
4. Model Governance and the Drift Dilemma
5. EHR Integration: Hooks, Workflows, and Reality
6. The Retrieval Augmentation Challenge
7. Latency, Safety, and the Clinical Moment
8. Economic Models and Sustainability
9. Regulatory Considerations and Risk Management
10. Future Directions and Conclusions
Introduction: The Promise and Peril of Intelligent CDS
Anyone who has spent time in a modern hospital or clinic knows the sound intimately - the constant symphony of beeps, alerts, and notifications that punctuate every clinical interaction. The average physician encounters between 150 to 300 clinical decision support alerts per day, with override rates consistently exceeding 90 percent across most healthcare systems. This is the legacy of first-generation CDS: well-intentioned but fundamentally flawed systems that mistake quantity for quality, noise for intelligence. The result has been a generation of clinicians trained to reflexively dismiss the very systems designed to help them, creating what researchers call "alert fatigue" but what might more accurately be described as "CDS learned helplessness."
The emergence of foundation models - large language models and their multimodal cousins - represents perhaps the most significant opportunity in decades to fundamentally reimagine clinical decision support. These models, trained on vast corpora of medical literature, clinical notes, and structured data, demonstrate an unprecedented ability to reason about complex clinical scenarios, synthesize information across multiple domains, and generate contextually appropriate recommendations. However, the gap between laboratory demonstrations and production healthcare deployments remains vast, filled with technical, regulatory, and practical challenges that the healthcare technology community has only begun to address.
The question facing health tech entrepreneurs and investors today is not whether foundation models will transform clinical decision support - they will - but rather how quickly we can solve the engineering and governance challenges that stand between promising prototypes and reliable, scalable systems that clinicians will actually trust and use. This transformation requires moving beyond the simplistic alert-based paradigm that has dominated CDS for the past two decades toward more sophisticated systems that understand context, timing, and clinical workflow at a granular level.
The stakes could not be higher. Healthcare systems worldwide face unprecedented pressures: aging populations, clinician shortages, rising costs, and increasing complexity of medical knowledge. The World Health Organization estimates that diagnostic errors affect 12 million adults annually in the United States alone, with missed diagnoses contributing to approximately 40,000 to 80,000 deaths each year. Meanwhile, the volume of medical knowledge continues to grow exponentially, with over 4,000 new clinical studies published daily. No human clinician can possibly stay current with this flood of information, creating an urgent need for intelligent systems that can synthesize and apply this knowledge at the point of care.
Foundation models offer the tantalizing possibility of creating CDS systems that function more like highly knowledgeable specialists than rule-based alarm systems. Instead of triggering alerts when blood pressure exceeds a threshold, these systems could analyze the complete clinical picture - patient history, current medications, recent labs, vital trends, and relevant literature - to provide nuanced, personalized recommendations that account for the full complexity of individual patient care. The technical challenges of building such systems are formidable, but the potential impact on patient outcomes and clinical efficiency justifies the substantial investment required.
The Foundation Model Revolution in Healthcare
The transformation of clinical decision support through foundation models represents more than an incremental improvement in existing systems - it constitutes a fundamental shift in how we conceptualize the relationship between artificial intelligence and clinical reasoning. Traditional CDS systems operate through explicitly programmed rules and decision trees, requiring domain experts to anticipate and codify every possible clinical scenario. Foundation models, by contrast, develop implicit understanding of clinical relationships through exposure to vast amounts of medical text and data, enabling them to reason about novel situations and make connections that may not be immediately apparent to human programmers.
The technical capabilities that make foundation models particularly suited for clinical applications extend far beyond simple text processing. Modern multimodal foundation models can simultaneously process clinical notes, laboratory results, imaging studies, vital signs, and medication histories, creating integrated representations of patient state that mirror the holistic thinking of experienced clinicians. GPT-4 and similar models have demonstrated performance on medical licensing examinations that exceeds the passing threshold by substantial margins, with recent studies showing accuracy rates between 85-90 percent on the United States Medical Licensing Examination (USMLE) Step examinations.
However, examination performance, while impressive, represents only a fraction of what foundation models must accomplish in real clinical environments. The ability to synthesize information across multiple data types and time scales, reason about uncertainty and incomplete information, and generate recommendations that account for individual patient preferences and constraints requires capabilities that go far beyond answering multiple-choice questions. Recent research from Stanford and other institutions has begun to demonstrate these more sophisticated capabilities, with foundation models showing the ability to identify subtle patterns in clinical data that may escape human attention.
The multimodal capabilities of next-generation foundation models present particularly compelling opportunities for clinical decision support. Models like GPT-4V and Claude-3 can analyze medical images alongside clinical text, potentially identifying correlations between radiological findings and patient symptoms that might not be apparent when these data sources are considered in isolation. Early studies have shown promising results in areas such as diabetic retinopathy screening, where foundation models can achieve diagnostic accuracy comparable to specialist ophthalmologists while providing detailed explanations of their reasoning process.
The ability of foundation models to maintain coherent reasoning across extended contexts - current models can process contexts of 100,000 to 200,000 tokens - enables them to consider comprehensive patient histories when making recommendations. This represents a fundamental advantage over traditional CDS systems, which typically operate on limited snapshots of patient data. A foundation model-based CDS system could potentially analyze a patient's complete medical history, including previous hospitalizations, medication trials, and treatment responses, to provide recommendations that account for the full trajectory of the patient's clinical journey.
Perhaps most importantly for clinical applications, foundation models demonstrate emergent capabilities in uncertainty quantification and explanation generation. Unlike traditional machine learning models that provide point predictions, foundation models can express confidence levels in their recommendations and provide detailed reasoning chains that clinicians can evaluate and critique. This transparency is essential for building trust in clinical environments, where the ability to understand and validate AI recommendations can mean the difference between life and death.
The economic implications of foundation model-based CDS are equally compelling. Traditional CDS development requires substantial investments in clinical expertise, rule development, and ongoing maintenance as medical knowledge evolves. Foundation models, once trained, can be fine-tuned for specific clinical domains and updated with new medical literature through relatively straightforward retraining processes. This could dramatically reduce the cost and complexity of maintaining current CDS systems while simultaneously improving their capabilities.
System Architecture: Cloud vs Edge in Clinical Environments
The architectural decisions surrounding foundation model deployment in clinical environments represent some of the most consequential choices facing health tech entrepreneurs today. The fundamental trade-off between cloud-based inference, which offers unlimited computational resources and seamless model updates, and edge deployment, which provides data sovereignty and consistent performance, reflects deeper tensions around privacy, reliability, and cost optimization in healthcare technology systems.
Cloud-based deployment models offer compelling advantages for foundation model inference. The computational requirements for running large language models efficiently - modern clinical models may require 40GB to 80GB of GPU memory for optimal performance - make cloud deployment an attractive option for healthcare organizations that lack substantial AI infrastructure. Amazon's HealthScribe service and Google's Med-Gemini platform demonstrate the potential of cloud-based approaches, offering pre-trained medical models accessible through API endpoints with per-token pricing models that can make advanced capabilities accessible to smaller healthcare organizations.
However, the reality of healthcare data governance creates significant complications for cloud-based approaches. HIPAA compliance requirements, while not explicitly prohibiting cloud processing of protected health information, create complex contractual and technical requirements that many healthcare organizations find challenging to navigate. The recent emphasis on data localization in healthcare - driven by both regulatory requirements and institutional risk management policies - has led many health systems to prefer on-premises solutions despite their higher infrastructure costs and complexity.
The latency characteristics of cloud versus edge deployment present another critical consideration. Clinical decision support systems must operate within the natural rhythm of clinical workflows, providing recommendations and insights at the moment of clinical decision-making rather than minutes or hours later. Network latency to cloud endpoints, even with optimized connections, typically ranges from 20-100 milliseconds for basic connectivity, before accounting for model inference time. For foundation models processing complex clinical scenarios, cloud inference times can range from 500 milliseconds to several seconds, potentially disrupting the flow of clinical care.
Edge deployment models, while requiring substantially greater upfront investment, offer more predictable performance characteristics. NVIDIA's Clara platform and similar solutions enable healthcare organizations to deploy foundation models on local hardware, providing inference latency in the 50-200 millisecond range that aligns well with clinical workflow requirements. The total cost of ownership for edge deployments, while higher initially, may prove more economical for large healthcare systems with high query volumes, particularly as GPU hardware costs continue to decline.
Hybrid architectures represent an increasingly attractive middle ground, enabling healthcare organizations to maintain sensitive operations on-premises while leveraging cloud resources for less critical applications. These architectures typically employ local models for real-time decision support during patient encounters, with cloud-based systems handling batch processing tasks such as population health analytics and model training. The technical complexity of managing hybrid deployments - including data synchronization, model versioning, and failover mechanisms - requires sophisticated DevOps capabilities that many healthcare organizations are still developing.
The containerization of foundation models through platforms like Docker and Kubernetes has simplified deployment across different infrastructure environments, enabling healthcare organizations to develop vendor-agnostic solutions that can operate across cloud and edge environments. Container orchestration platforms designed specifically for healthcare, such as Red Hat OpenShift for Healthcare, provide additional security and compliance features that address many of the concerns associated with deploying AI systems in regulated environments.
Security considerations add another layer of complexity to architectural decisions. Edge deployments offer inherent data protection through isolation but require robust physical security and access controls. Cloud deployments benefit from the security expertise of major cloud providers but introduce additional attack surfaces and data transmission risks. The recent emphasis on homomorphic encryption and secure multi-party computation in healthcare AI applications suggests potential future architectures that could provide cloud-scale computational resources while maintaining data privacy guarantees.
Model Governance and the Drift Dilemma
The challenge of maintaining consistent, reliable performance from foundation models in clinical environments represents one of the most underappreciated technical challenges facing the healthcare AI community. Unlike traditional software systems, foundation models exhibit complex, often unpredictable changes in behavior as they encounter new data patterns, receive updates, or operate in environments different from their training conditions. This phenomenon, known as model drift, poses particular challenges in healthcare settings where consistency and reliability are paramount.
Clinical model drift manifests in several distinct forms, each requiring different monitoring and mitigation strategies. Data drift occurs when the statistical properties of input data change over time - for example, as patient populations evolve, new diagnostic codes are introduced, or clinical documentation practices change. Concept drift represents changes in the underlying relationships between inputs and desired outputs, such as when new treatment guidelines modify the optimal clinical recommendations for specific patient presentations. Model drift, in the narrow sense, refers to changes in model behavior that occur independently of data changes, potentially due to updates in underlying model weights or infrastructure modifications.
The healthcare environment presents particularly challenging conditions for model governance due to the heterogeneity of clinical data and the rapid evolution of medical practice. Electronic health record systems capture clinical information in numerous formats - structured data fields, clinical notes, imaging studies, laboratory results - with significant variation in documentation practices across different providers, specialties, and institutions. Foundation models trained on data from one healthcare system may exhibit degraded performance when deployed in different environments, requiring sophisticated adaptation strategies.
Continuous monitoring frameworks for clinical foundation models must track performance across multiple dimensions simultaneously. Traditional machine learning monitoring focuses primarily on prediction accuracy and system performance metrics. Clinical applications require additional monitoring of safety-critical outcomes, adherence to clinical guidelines, consistency with established medical knowledge, and alignment with institutional policies and preferences. The development of comprehensive monitoring dashboards that provide real-time visibility into model performance across these multiple dimensions represents a significant technical challenge.
Version control and rollback capabilities become particularly critical in clinical environments where model updates could potentially impact patient care. Unlike consumer applications where gradual performance degradation might be acceptable, clinical decision support systems require the ability to quickly revert to previous model versions if performance issues are detected. This requires sophisticated model versioning infrastructure that can maintain multiple model versions simultaneously while providing seamless switching capabilities.
The temporal aspects of model governance in healthcare add additional complexity. Clinical guidelines and best practices evolve continuously, requiring foundation models to adapt to new evidence and recommendations while maintaining consistency with established care patterns. The lag time between publication of new clinical evidence and its integration into AI systems could create situations where AI recommendations lag behind current best practices, potentially compromising patient care quality.
Federated learning approaches offer promising solutions to some governance challenges by enabling model updates without centralizing sensitive clinical data. Healthcare organizations can contribute to model improvement while maintaining data sovereignty, creating collaborative governance frameworks that benefit from collective clinical experience. However, federated learning introduces additional technical complexity around communication protocols, privacy preservation, and consensus mechanisms that many healthcare organizations lack the technical expertise to implement effectively.
The regulatory landscape surrounding model governance continues to evolve rapidly, with FDA guidance on AI/ML-based medical devices emphasizing the importance of predetermined change control plans and continuous monitoring capabilities. Healthcare organizations deploying foundation models for clinical decision support must develop governance frameworks that satisfy regulatory requirements while maintaining operational flexibility. This often requires close collaboration between clinical, technical, and regulatory teams to ensure that governance processes support both innovation and compliance objectives.
EHR Integration: Hooks, Workflows, and Reality
The integration of foundation model-based clinical decision support systems with existing electronic health record platforms represents one of the most technically challenging and practically important aspects of deploying AI in healthcare environments. The reality of clinical workflow integration extends far beyond simple API connections to encompass complex questions of user experience design, clinical workflow optimization, and change management that determine whether sophisticated AI capabilities actually improve patient care outcomes.
Epic and Cerner, which collectively serve approximately 70 percent of hospital beds in the United States, have developed extensive hook systems that enable third-party applications to integrate with clinical workflows. Epic's App Orchard marketplace and Cerner's Developer Network provide access to numerous integration points - from simple data reads to complex workflow interruptions - but the practical reality of implementing foundation model-based CDS through these systems reveals significant challenges that are not immediately apparent from vendor documentation.
The Epic hooks system provides multiple integration patterns for clinical decision support applications. Best Practice Advisories (BPAs) enable real-time alerts and recommendations triggered by specific clinical events or data patterns. SmartForms and SmartPhrases allow integration of AI-generated content directly into clinical documentation workflows. The more recent Smart on FHIR framework enables web-based applications to access clinical data and present information within the Epic user interface. However, each integration pattern introduces different latency requirements, user experience constraints, and technical limitations that significantly impact the feasibility of foundation model integration.
Real-time integration through Epic BPAs requires AI recommendations to be generated within extremely tight time constraints - typically 200-500 milliseconds from trigger event to recommendation display. This latency requirement effectively precludes the use of large foundation models for real-time decision support unless sophisticated caching and prediction strategies are employed. Many successful implementations rely on hybrid approaches where lightweight models provide immediate feedback while foundation models generate more detailed recommendations asynchronously.
The user experience implications of EHR integration often prove more challenging than the technical aspects. Clinicians have developed highly optimized workflows around existing EHR interfaces, with experienced users capable of navigating complex patient records in seconds through memorized click patterns and keyboard shortcuts. Foundation model-based CDS systems must integrate seamlessly into these established workflows without disrupting clinical efficiency or introducing additional cognitive load.
The concept of "clinical flow state" - the focused, efficient mental state that characterizes effective clinical work - provides a useful framework for evaluating CDS integration strategies. Interventions that interrupt clinical flow or require context switching between different information systems often fail to achieve adoption despite superior technical capabilities. Successful foundation model integrations typically emphasize subtle augmentation of existing workflows rather than replacement of established patterns.
FHIR R4 compliance has become increasingly important for EHR integration strategies, providing standardized data models and API interfaces that enable more portable integration approaches. However, the reality of FHIR implementation varies significantly across different EHR vendors and healthcare organizations. While Epic and Cerner provide robust FHIR APIs, the completeness and consistency of data available through these APIs often falls short of what is accessible through native integration approaches.
The emerging trend toward EHR-agnostic integration strategies, enabled by platforms like 1up Health and Redox, offers potential solutions to vendor lock-in challenges. These platforms provide abstraction layers that enable AI applications to work across multiple EHR systems through standardized interfaces. However, the abstraction process inevitably results in some loss of functionality and performance compared to native integrations, requiring careful evaluation of trade-offs between portability and capability.
Mobile integration presents additional opportunities and challenges for foundation model-based CDS. Clinicians increasingly rely on mobile devices for clinical communication and information access, creating opportunities for AI systems to provide recommendations and insights outside of traditional desktop-based workflows. However, mobile integration introduces additional constraints around battery life, network connectivity, and user interface design that require specialized technical approaches.
The Retrieval Augmentation Challenge
The promise of retrieval-augmented generation (RAG) for clinical decision support lies in its potential to combine the broad knowledge and reasoning capabilities of foundation models with up-to-date, institution-specific clinical guidelines and protocols. However, the technical challenges of implementing effective RAG systems in healthcare environments - particularly while maintaining the sub-200 millisecond latency requirements of real-time clinical decision support - represent some of the most complex engineering problems in healthcare AI today.
Traditional RAG architectures rely on vector databases to store embeddings of relevant documents, enabling rapid retrieval of contextually relevant information to augment foundation model prompts. In healthcare applications, this approach must accommodate multiple types of clinical knowledge: published clinical guidelines from professional societies, institutional protocols and order sets, local formulary restrictions, population-specific considerations, and individual patient history. The heterogeneity of these data sources creates significant challenges for embedding generation and retrieval optimization.
The latency requirements of clinical decision support create severe constraints on RAG system architecture. A typical RAG pipeline involves several sequential steps: query processing, embedding generation, vector similarity search, document retrieval, context assembly, and foundation model inference. Each step contributes to total latency, with the cumulative delay often exceeding acceptable thresholds for real-time clinical applications. Achieving sub-200 millisecond performance requires aggressive optimization at every level of the system stack.
Vector database selection for clinical RAG systems involves complex trade-offs between query latency, indexing performance, and memory requirements. Specialized vector databases like Pinecone and Weaviate offer optimized performance for similarity search operations but introduce additional infrastructure complexity and cost. In-memory solutions like Faiss provide extremely low latency but require careful capacity planning and may not scale effectively to large clinical knowledge bases. The recent emergence of hybrid database systems that combine vector search with traditional relational capabilities, such as PostgreSQL with pgvector extensions, offers promising middle-ground approaches.
The chunking strategy for clinical documents significantly impacts both retrieval accuracy and system performance. Clinical guidelines often contain complex hierarchical information - recommendations that depend on specific patient populations, contraindications, drug interaction warnings, and dosing adjustments - that must be preserved during the chunking process. Simple sentence-based or paragraph-based chunking strategies may break important contextual relationships, while more sophisticated semantic chunking approaches introduce additional processing overhead that impacts latency performance.
Hybrid retrieval strategies that combine dense vector search with sparse keyword-based methods have shown promising results for clinical applications. Dense retrieval excels at finding semantically similar content but may miss exact matches for specific drug names, diagnostic codes, or clinical terminology. Sparse retrieval provides precise matching for specific terms but lacks the semantic understanding necessary for complex clinical reasoning. Combining these approaches through learned ranking models can improve retrieval quality while maintaining acceptable performance characteristics.
The caching and pre-computation of common clinical scenarios represents a critical optimization strategy for achieving acceptable latency in RAG-based systems. Clinical decision support often involves recurring patterns - common drug interactions, standard diagnostic workups, routine preventive care recommendations - that can be pre-computed and cached to avoid real-time retrieval operations. However, effective caching requires sophisticated prediction of likely clinical scenarios and careful invalidation strategies to ensure cached content remains current.
Multi-stage retrieval architectures offer another approach to balancing quality and performance in clinical RAG systems. Initial retrieval stages use fast, approximate methods to identify potentially relevant documents, while subsequent stages apply more sophisticated ranking and filtering approaches to select the most appropriate content for context assembly. This approach enables systems to maintain high recall in the initial retrieval phase while optimizing precision in the final context selection phase.
The integration of structured clinical data with unstructured text retrieval presents unique challenges and opportunities. Laboratory results, vital signs, medication lists, and diagnostic codes provide valuable context for clinical decision support but require different indexing and retrieval strategies than text-based documents. Recent advances in multimodal embeddings that can represent both structured and unstructured data within unified vector spaces show promise for addressing these challenges.
Latency, Safety, and the Clinical Moment
The temporal dynamics of clinical decision-making create unique constraints for AI system design that extend far beyond simple performance optimization. The concept of the "clinical moment" - the brief window of time during which a clinical decision must be made - defines the operational requirements for foundation model-based decision support systems. Understanding and optimizing for these temporal constraints often determines the difference between AI systems that enhance clinical care and those that remain unused despite superior technical capabilities.
Clinical decision-making operates across multiple time scales simultaneously. Immediate decisions - such as medication dosing during procedures or emergency interventions - require AI recommendations within seconds. Near-term decisions - such as diagnostic workups or treatment modifications - may accommodate latency measured in minutes. Strategic decisions - such as discharge planning or chronic disease management - can incorporate AI insights generated over hours or days. Effective CDS systems must recognize these different temporal requirements and optimize their response characteristics accordingly.
The relationship between latency and clinical utility follows a non-linear pattern that reflects the cognitive load associated with clinical decision-making. Response times under 100 milliseconds feel instantaneous to clinicians and integrate seamlessly into clinical thought processes. Latency between 100-500 milliseconds remains acceptable for most clinical applications but begins to create noticeable delays. Response times exceeding 1-2 seconds often result in task switching and loss of clinical context, significantly reducing the utility of AI recommendations even when they are technically superior.
Safety considerations in clinical AI systems require fundamentally different approaches to system reliability than consumer applications. The potential consequences of AI system failures in healthcare - delayed diagnoses, inappropriate medications, missed critical interventions - demand fault tolerance and graceful degradation capabilities that exceed typical software engineering standards. Foundation model-based CDS systems must maintain safe default behaviors when inference systems become unavailable, network connectivity fails, or model performance degrades below acceptable thresholds.
The implementation of safety nets for clinical AI systems often involves multi-layered approaches that combine technological safeguards with human oversight mechanisms. Automatic confidence scoring enables systems to flag recommendations that fall below reliability thresholds, ensuring that uncertain AI outputs are clearly identified for human review. Consistency checking against established clinical guidelines helps identify potentially dangerous recommendations before they reach clinicians. Real-time monitoring of system performance enables rapid detection and mitigation of safety issues.
The concept of "fail-safe" versus "fail-secure" design philosophies takes on particular importance in clinical applications. Fail-safe systems default to established clinical protocols when AI components malfunction, ensuring continuity of care even when advanced decision support becomes unavailable. Fail-secure systems may choose to restrict access to AI capabilities when safety constraints cannot be guaranteed, potentially impacting clinical efficiency but prioritizing patient safety above all other considerations.
Latency optimization for clinical AI systems requires sophisticated understanding of the complete system architecture, from data ingestion through recommendation delivery. Database query optimization becomes critical when AI systems must access real-time clinical data from EHR systems, laboratory information systems, and other clinical data sources. Connection pooling, query caching, and database replication strategies can significantly impact the speed of data retrieval operations that precede AI inference.
Model optimization techniques specifically tailored for clinical applications have emerged as a critical area of technical development. Knowledge distillation enables smaller, faster models to approximate the performance of larger foundation models while meeting clinical latency requirements. Quantization and pruning techniques reduce model memory requirements and inference time while preserving clinical accuracy. Specialized inference engines optimized for medical language understanding can provide significant performance improvements over general-purpose AI inference platforms.
The geographic distribution of clinical AI infrastructure introduces additional latency considerations that vary significantly across different healthcare delivery models. Large health systems with centralized data centers may achieve optimal performance through dedicated AI infrastructure co-located with clinical data systems. Smaller healthcare organizations relying on cloud-based or shared infrastructure must carefully optimize network connectivity and data transfer protocols to minimize latency impacts.
Economic Models and Sustainability
The economic landscape surrounding foundation model-based clinical decision support presents complex challenges that extend far beyond simple cost-benefit analyses. Healthcare organizations operate under unique financial constraints - value-based care contracts, regulatory compliance requirements, capital equipment depreciation schedules - that significantly influence technology adoption decisions. Understanding these economic dynamics is essential for developing sustainable business models that can support the substantial investments required for advanced AI capabilities in healthcare.
The total cost of ownership for foundation model-based CDS systems includes several categories of expenses that may not be immediately apparent to healthcare technology entrepreneurs. Infrastructure costs encompass not only computational resources for model inference but also data storage, network connectivity, security systems, and backup capabilities required to support production clinical applications. Human resources costs include not only technical staff for system maintenance and optimization but also clinical staff training, change management support, and ongoing governance activities.
The computational economics of foundation model inference create particular challenges for healthcare applications. Current generation clinical foundation models require substantial computational resources - often 40-80 GB of GPU memory and significant processing power for real-time inference. The cost of these computational resources, whether procured through cloud services or on-premises infrastructure, must be distributed across the volume of clinical decisions supported by the system. For large health systems processing thousands of clinical decisions daily, per-decision costs may be reasonable. For smaller healthcare organizations with lower query volumes, the fixed costs of foundation model infrastructure may be prohibitive.
Value-based care contracts, which tie healthcare reimbursement to patient outcomes rather than volume of services provided, create particularly interesting economic dynamics for clinical AI investments. CDS systems that demonstrably improve diagnostic accuracy, reduce medical errors, or optimize treatment selection can generate substantial economic value through improved patient outcomes and reduced liability exposure. However, quantifying these benefits requires sophisticated measurement systems and long-term outcome tracking that many healthcare organizations lack.
The subscription-based pricing models common in healthcare technology - where organizations pay recurring fees for access to AI capabilities - offer advantages for both vendors and customers by spreading costs over time and enabling continuous improvement of AI systems. However, these models introduce ongoing operational expenses that healthcare organizations must incorporate into their long-term financial planning. The predictability of subscription costs can aid in budget planning, but the cumulative expense over time may exceed the cost of alternative approaches.
The economic impact of clinical AI systems extends beyond direct cost savings to encompass improvements in clinical efficiency and care quality that may be difficult to quantify but create substantial value. Reduced time spent on routine clinical tasks enables clinicians to focus on more complex cases and patient interaction. Improved diagnostic accuracy reduces the costs associated with medical errors, malpractice claims, and unnecessary procedures. Enhanced care coordination and treatment optimization can improve patient satisfaction scores that impact reimbursement under value-based care models.
The development of economic measurement frameworks for clinical AI represents a critical need in the healthcare technology community. Traditional return-on-investment calculations may not capture the full value proposition of AI systems that improve patient safety, enhance clinical decision-making, or reduce cognitive load on clinical staff. More sophisticated economic models that account for risk reduction, quality improvements, and long-term outcome benefits are necessary to support investment decisions in healthcare AI technologies.
The scalability economics of foundation model-based CDS systems present both opportunities and challenges. Once deployed, AI systems can potentially serve unlimited numbers of clinical decisions with minimal incremental cost, creating attractive unit economics for high-volume applications. However, achieving the scale necessary to realize these economics may require substantial upfront investments in infrastructure, integration, and clinical validation that many healthcare organizations cannot support independently.
Regulatory Considerations and Risk Management
The regulatory landscape surrounding AI-enabled clinical decision support continues to evolve rapidly, creating both opportunities and challenges for healthcare technology entrepreneurs. The FDA's approach to AI/ML-based medical devices has shifted toward more flexible frameworks that accommodate the iterative nature of machine learning systems, but significant uncertainties remain around approval pathways, liability frameworks, and ongoing compliance requirements for foundation model-based clinical applications.
The FDA's 2019 discussion paper on AI/ML-based Software as Medical Device established important precedents for regulating AI systems that continuously learn and adapt. The concept of a "predetermined change control plan" enables AI systems to receive initial approval for specific types of modifications and improvements without requiring full regulatory review for each update. This framework is particularly relevant for foundation model-based CDS systems, which may require frequent updates to incorporate new clinical knowledge or improve performance based on real-world usage data.
However, the application of existing regulatory frameworks to foundation models presents novel challenges that regulators are still learning to address. Traditional medical device regulation assumes relatively static systems with well-defined inputs, outputs, and failure modes. Foundation models, by contrast, exhibit emergent behaviors, complex interaction effects, and the potential for unexpected failure modes that may not be apparent during initial validation studies. The black-box nature of large language models creates additional challenges for regulatory review processes that traditionally require detailed understanding of system behavior and failure mechanisms.
The distinction between decision support and diagnostic functions has significant regulatory implications for foundation model applications. Software that provides information to clinicians for their consideration - traditional decision support - typically falls under less stringent regulatory requirements than software that provides specific diagnostic conclusions. However, foundation models capable of sophisticated clinical reasoning may blur these traditional boundaries, potentially providing recommendations that are functionally equivalent to diagnostic determinations even when presented as decision support.
Risk management frameworks for clinical AI systems must address multiple categories of potential harm that extend beyond traditional software failure modes. Clinical risks include missed diagnoses, inappropriate treatment recommendations, and safety alerts that go unheeded due to alert fatigue. Technical risks encompass system downtime, data breaches, model drift, and integration failures. Operational risks include user error, workflow disruption, and dependency on vendor-provided services that may become unavailable.
The development of comprehensive risk mitigation strategies requires close collaboration between clinical, technical, and legal teams to identify potential failure modes and implement appropriate safeguards. Clinical validation studies must demonstrate not only the accuracy of AI recommendations but also their impact on clinical workflows, decision-making processes, and patient outcomes. Post-market surveillance systems must monitor AI system performance in real-world clinical environments and detect degradation or unexpected behaviors that could compromise patient safety.
Liability considerations for AI-enabled clinical decision support create complex legal questions that the healthcare industry is still learning to address. Traditional medical malpractice frameworks assume human decision-makers who can be held accountable for clinical judgments. The introduction of AI systems that influence clinical decisions creates questions about liability distribution between healthcare providers, technology vendors, and the institutions that deploy these systems. Professional liability insurance policies may not adequately cover claims related to AI system failures or inappropriate recommendations.
The international regulatory landscape adds additional complexity for healthcare AI companies seeking to deploy foundation model-based systems across multiple jurisdictions. The European Union's Medical Device Regulation (MDR) and proposed AI Act create different requirements than FDA frameworks, while emerging regulations in Asia-Pacific markets introduce additional compliance considerations. Companies developing global healthcare AI solutions must navigate these diverse regulatory requirements while maintaining consistent safety and performance standards.
Data governance requirements for clinical AI systems extend beyond traditional HIPAA compliance to encompass sophisticated technical and administrative safeguards for AI training data, model artifacts, and inference processes. The use of clinical data for AI training requires careful attention to patient consent, data de-identification, and minimum necessary standards. The storage and processing of model weights and intermediate representations may create new categories of protected information that require specialized security measures.
Future Directions and Conclusions
The transformation of clinical decision support through foundation models represents one of the most significant opportunities in healthcare technology today, but realizing this potential requires addressing fundamental challenges that extend far beyond current technical capabilities. The next five years will likely determine whether foundation model-based CDS systems become indispensable tools that enhance clinical care or remain promising technologies that fail to achieve meaningful adoption due to unresolved implementation challenges.
The technical trajectory of foundation models suggests several developments that could significantly impact clinical applications. Multimodal models capable of processing clinical text, laboratory data, imaging studies, and physiological signals within unified architectures promise more comprehensive clinical reasoning capabilities. Specialized medical foundation models trained exclusively on clinical data may offer superior performance for healthcare applications while addressing some of the safety and reliability concerns associated with general-purpose models.
The emergence of smaller, more efficient foundation models optimized for specific clinical tasks could address many of the latency and cost challenges that currently limit deployment options. Recent advances in model distillation, quantization, and specialized inference hardware suggest that clinically capable foundation models may soon operate effectively on standard healthcare IT infrastructure without requiring specialized AI hardware or cloud-based inference services.
The integration of foundation models with existing clinical workflow tools represents a critical area for continued development. Rather than creating new user interfaces that compete with established EHR systems, the most successful implementations may focus on seamless augmentation of existing clinical workflows through intelligent automation, context-aware recommendations, and proactive identification of clinical opportunities.
The development of industry standards for clinical AI systems will likely accelerate as foundation model deployments become more common. Standardized approaches to model validation, performance monitoring, risk management, and regulatory compliance could reduce the barriers to adoption while ensuring consistent safety and quality standards across different implementations.
The economic models supporting clinical AI development continue to evolve as healthcare organizations gain experience with AI deployments and develop more sophisticated approaches to measuring value and return on investment. The shift toward value-based care contracts creates increasing incentives for healthcare organizations to invest in technologies that demonstrably improve patient outcomes, potentially providing sustainable funding models for advanced AI capabilities.
The regulatory landscape will likely continue evolving toward more flexible frameworks that can accommodate the unique characteristics of AI systems while maintaining appropriate safety standards. The development of regulatory guidance specifically tailored to foundation model applications could provide greater clarity for healthcare technology entrepreneurs while establishing clear expectations for safety and performance validation.
However, significant challenges remain that could limit the pace of foundation model adoption in healthcare. The shortage of technical talent with both AI expertise and healthcare domain knowledge continues to constrain development and deployment capabilities. The complexity of healthcare IT environments and the conservative risk tolerance of healthcare organizations create additional barriers to adoption that may prove more challenging than purely technical obstacles.
The question of whether foundation model-based clinical decision support will fulfill its transformative potential ultimately depends on the healthcare technology community's ability to solve these implementation challenges while maintaining focus on genuine clinical value rather than technological novelty. The organizations that succeed in this endeavor will likely be those that combine deep technical expertise with sophisticated understanding of clinical workflows, regulatory requirements, and healthcare economics.
The opportunity before us is unprecedented: to create clinical decision support systems that genuinely augment human clinical reasoning rather than simply generating alerts that clinicians ignore. Foundation models provide the technical foundation necessary to realize this vision, but success will require sustained effort across technical, clinical, regulatory, and economic dimensions. The healthcare technology entrepreneurs and investors who can navigate this complexity while maintaining unwavering focus on improving patient care outcomes will define the future of clinical decision support for decades to come.
The transformation will not happen overnight, and the path forward will likely include false starts, technological dead ends, and unexpected challenges that require creative solutions. However, the potential impact on patient care quality, clinical efficiency, and healthcare sustainability justifies the substantial investment and effort required to overcome these obstacles. The convergence of increasingly capable foundation models, maturing healthcare IT infrastructure, and growing economic pressures on healthcare delivery creates a unique window of opportunity that may not persist indefinitely.
The successful implementation of foundation model-based clinical decision support will require unprecedented collaboration between technologists, clinicians, regulators, and healthcare administrators. This collaboration must go beyond superficial consultation to encompass deep integration of clinical expertise into every aspect of system design, deployment, and governance. The organizations that can foster these collaborative relationships while maintaining rapid innovation cycles will likely emerge as leaders in the next generation of healthcare technology.
The international implications of clinical AI development add another dimension to these considerations. Healthcare delivery models vary significantly across different countries and regions, creating both challenges and opportunities for foundation model applications. The systems developed for the highly digitized, EHR-centric environment of United States healthcare may require substantial adaptation for deployment in healthcare systems with different technological infrastructures, regulatory frameworks, and clinical practices.
The educational implications of widespread clinical AI adoption represent both an opportunity and a responsibility for the healthcare technology community. As foundation model-based systems become more capable and ubiquitous, the training of future clinicians must evolve to include sophisticated understanding of AI capabilities, limitations, and appropriate use cases. Medical education curricula that fail to address these considerations may produce clinicians ill-equipped to work effectively with AI-augmented clinical environments.
The ethical considerations surrounding clinical AI deployment continue to evolve as these systems become more sophisticated and influential in clinical decision-making. Questions of algorithmic bias, health equity, patient autonomy, and the appropriate balance between human judgment and machine recommendations require ongoing attention from both technologists and ethicists. The development of ethical frameworks specifically tailored to foundation model applications in healthcare represents an urgent need that the technology community must address proactively.
The data network effects created by successful clinical AI implementations could fundamentally alter competitive dynamics in healthcare technology. Organizations that achieve scale in clinical AI deployment may benefit from continuous improvement cycles where increased usage generates better training data, leading to improved model performance, which drives further adoption. Understanding and leveraging these network effects while avoiding potential monopolistic outcomes will require careful consideration of market structure and competitive dynamics.
The environmental implications of large-scale foundation model deployment in healthcare deserve consideration as these systems become more prevalent. The computational requirements for training and operating foundation models create substantial energy consumption that healthcare organizations increasingly consider in their sustainability planning. The development of more efficient model architectures and inference systems represents both an environmental imperative and a competitive advantage for healthcare AI companies.
Looking ahead, the most successful foundation model implementations in healthcare will likely be those that seamlessly integrate into the natural flow of clinical work while providing demonstrable value that justifies their complexity and cost. The systems that achieve widespread adoption will probably be those that make clinicians more effective rather than those that attempt to replace human judgment with artificial intelligence.
The measurement of success for clinical AI initiatives must extend beyond traditional technology metrics to encompass clinical outcomes, user satisfaction, and healthcare system performance indicators. The development of comprehensive evaluation frameworks that can capture the full impact of AI systems on healthcare delivery will be essential for guiding future development efforts and investment decisions.
The foundation model revolution in clinical decision support represents more than a technological advancement - it constitutes a fundamental reimagining of how knowledge, experience, and clinical reasoning can be augmented and democratized across healthcare delivery systems. The technical challenges are substantial, the regulatory landscape is complex, and the economic models are still evolving. However, the potential to improve patient care quality, reduce medical errors, and enhance clinical efficiency provides compelling justification for the sustained effort required to overcome these obstacles.
The organizations and individuals who successfully navigate this transformation will not only achieve significant commercial success but will also contribute to one of the most important advances in healthcare delivery since the introduction of antibiotics or modern anesthesia. The opportunity before us is not merely to build better software, but to fundamentally enhance human clinical reasoning and improve health outcomes for millions of patients worldwide.
The next chapter in healthcare technology will be written by those who can combine technical excellence with deep clinical understanding, regulatory sophistication with rapid innovation, and commercial success with genuine commitment to improving patient care. The foundation models are ready. The question now is whether the healthcare technology community is prepared to meet the challenge of implementing them effectively, safely, and sustainably in the complex environment of modern clinical practice.
The future of clinical decision support lies not in replacing human clinical judgment but in augmenting it with artificial intelligence systems that can process vast amounts of information, identify subtle patterns, and provide contextually appropriate recommendations at the moment of clinical decision-making. Foundation models provide the technical foundation for this vision, but realizing it will require the best efforts of technologists, clinicians, regulators, and healthcare leaders working together toward the common goal of better patient care.justifies the substantial investment and effort required to overcome these obstacles. The convergence of increasingly capable foundation models, maturing healthcare IT infrastructure, and growing economic pressures on healthcare delivery creates a unique window of opportunity that may not persist indefinitely.
The successful implementation of foundation model-based clinical decision support will require unprecedented collaboration between technologists, clinicians, regulators, and healthcare administrators. This collaboration must go beyond superficial consultation to encompass deep integration of clinical expertise into every aspect of system design, deployment, and governance. The organizations that can foster these collaborative relationships while maintaining rapid innovation cycles will likely emerge as leaders in the next generation of healthcare technology.
The international implications of clinical AI development add another dimension to these considerations. Healthcare delivery models vary significantly across different countries and regions, creating both challenges and opportunities for foundation model applications. The systems developed for the highly digitized, EHR-centric environment of United States healthcare may require substantial adaptation for deployment in healthcare systems with different technological infrastructures, regulatory frameworks, and clinical practices.
The educational implications of widespread clinical AI adoption represent both an opportunity and a responsibility for the healthcare technology community. As foundation model-based systems become more capable and ubiquitous, the training of future clinicians must evolve to include sophisticated understanding of AI capabilities, limitations, and appropriate use cases. Medical education curricula that fail to address these considerations may produce clinicians ill-equipped to work effectively with AI-augmented clinical environments.
The ethical considerations surrounding clinical AI deployment continue to evolve as these systems become more sophisticated and influential in clinical decision-making. Questions of algorithmic bias, health equity, patient autonomy, and the appropriate balance between human judgment and machine recommendations require ongoing attention from both technologists and ethicists. The development of ethical frameworks specifically tailored to foundation model applications in healthcare represents an urgent need that the technology community must address proactively.
The data network effects created by successful clinical AI implementations could fundamentally alter competitive dynamics in healthcare technology. Organizations that achieve scale in clinical AI deployment may benefit from continuous improvement cycles where increased usage generates better training data, leading to improved model performance, which drives further adoption. Understanding and leveraging these network effects while avoiding potential monopolistic outcomes will require careful consideration of market structure and competitive dynamics.
The environmental implications of large-scale foundation model deployment in healthcare deserve consideration as these systems become more prevalent. The computational requirements for training and operating foundation models create substantial energy consumption that healthcare organizations increasingly consider in their sustainability planning. The development of more efficient model architectures and inference systems represents both an environmental imperative and a competitive advantage for healthcare AI companies.
Looking ahead, the most successful foundation model implementations in healthcare will likely be those that seamlessly integrate into the natural flow of clinical work while providing demonstrable value that justifies their complexity and cost. The systems that achieve widespread adoption will probably be those that make clinicians more effective rather than those that attempt to replace human judgment with artificial intelligence.
The measurement of success for clinical AI initiatives must extend beyond traditional technology metrics to encompass clinical outcomes, user satisfaction, and healthcare system performance indicators. The development of comprehensive evaluation frameworks that can capture the full impact of AI systems on healthcare delivery will be essential for guiding future development efforts and investment decisions.
The foundation model revolution in clinical decision support represents more than a technological advancement - it constitutes a fundamental reimagining of how knowledge, experience, and clinical reasoning can be augmented and democratized across healthcare delivery systems. The technical challenges are substantial, the regulatory landscape is complex, and the economic models are still evolving. However, the potential to improve patient care quality, reduce medical errors, and enhance clinical efficiency provides compelling justification for the sustained effort required to overcome these obstacles.
The organizations and individuals who successfully navigate this transformation will not only achieve significant commercial success but will also contribute to one of the most important advances in healthcare delivery since the introduction of antibiotics or modern anesthesia. The opportunity before us is not merely to build better software, but to fundamentally enhance human clinical reasoning and improve health outcomes for millions of patients worldwide.
The next chapter in healthcare technology will be written by those who can combine technical excellence with deep clinical understanding, regulatory sophistication with rapid innovation, and commercial success with genuine commitment to improving patient care. The foundation models are ready. The question now is whether the healthcare technology community is prepared to meet the challenge of implementing them effectively, safely, and sustainably in the complex environment of modern clinical practice.
The future of clinical decision support lies not in replacing human clinical judgment but in augmenting it with artificial intelligence systems that can process vast amounts of information, identify subtle patterns, and provide contextually appropriate recommendations at the moment of clinical decision-making. Foundation models provide the technical foundation for this vision, but realizing it will require the best efforts of technologists, clinicians, regulators, and healthcare leaders working together toward the common goal of better patient care.